are endergonic reactions spontaneous
An endergonic reaction will not take place on its own without the transfer of energy into the reaction or increase of entropy somewhere else. Web Endergonic Reactions Endergonic reactions may also be called an unfavorable reaction or nonspontaneous reaction.
 |
| What Is An Endergonic Reaction Sciencing |
If for a certain reaction it is satisfied that ΔG 0 then it will not be.
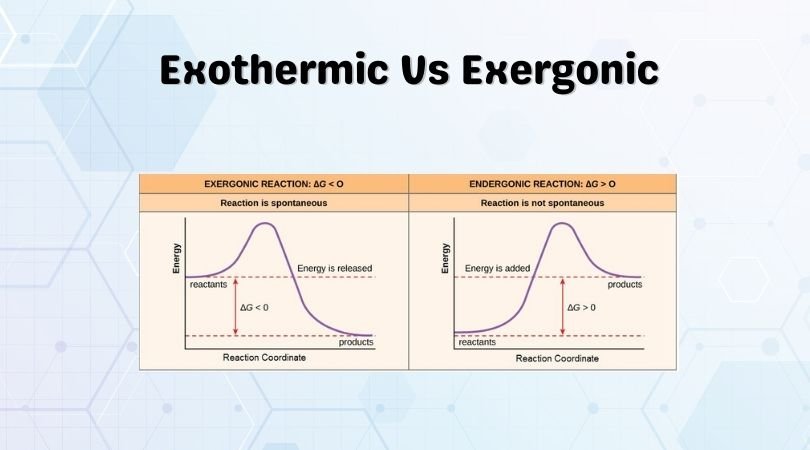
. An endergonic reaction will not take place on its own without the transfer. Web Every endergonic reaction is associated with an increase in the Gibbs free energy of the system. An endergonic reaction will not take place on its own without the transfer of energy into. The reaction requires more.
Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Web What is an Endergonic Reaction. Web The key difference between endergonic and exergonic is that endergonic reactions are non-spontaneous and unfavourable whereas exergonic reactions are. Web An engergonic reaction is one that has a dG change in free energy in order to happen.
An exergonic reaction is a reaction that releases free. Web This information is very valuable because such spontaneous reactions can be tapped to do useful work such as in metabolism. Burning of a log is another example of an exergonic reaction. Example of such reaction is the combustion of sodium when exposed to oxygen present in the air.
What makes an exergonic reaction. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. That depends on conditions. All physical and chemical systems in the universe follow the second law of thermodynamics and proceed in a downhill ie exergonic direction.
Web Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy. Web Endergonic reactions are nonspontaneous and require energy to occur. Here asking the same question for exothermic reactions but not all exergonic reactions are exothermic. These reactions occur spontaneously.
How they compare with Exothermic and Endothermic While the words Endergonic and. Web There are some questions eg. Web Endergonic reactions are nonspontaneous. It is not spontaneous.
These chemical reactions are what we refer to as endergonic reactions and they are non. Web The exergonic reaction is an irreversible reaction which occurs spontaneously in nature. Occur without insertion of energy or catalyst naturally. An exergonic reaction is a chemical reaction where the change in the free energy is negative there is a net release.
Web The main difference between exergonic reactions and endergonic reactions is that exergonic reactions are spontaneous ie. The progress of the reaction is shown by the line. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Thus left to itself any physical or chemical system will proceed according to the second law of thermodynamics in a direction that tends to lower the free energy of the system and thus to expend energy in the form of work.
Web In an endergonic reaction the free energy of the products is higher than the free energy of the reactants G 0. Web What is needed to reverse the process of spontaneous. If the ΔG 0. Reactions with a positive G G 0 on the other.
Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Web Conversely endergonic reactions require energy beyond activation energy to occur. Endergonic reaction is known as nonspontaneous reaction in thermodynamics or physical chemistry. The change of Gibbs free energy ΔG during an endergonic reaction.
Reaction coordinate diagrams of exergonic and endergonic reactions. The sum of the ΔG values of the two. It means it is ready or eager to occur with very little external stimuli. Energy is stored in the products so the reaction is not spontaneous and additional energy must be supplied to make the reaction proceed.
Change of Gibbs free energy for an. Web We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in. It may be spontaneous at certain temperatures or so. Web Thus we can think of the products of these reactions as energy-storing molecules.
 |
| Endergonic Vs Exergonic Reactions And Examples |
 |
| Chapter 6 Diagram Quizlet |
:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png) |
| Endergonic Vs Exergonic Reactions And Processes |
 |
| Thermodynamics Difference Between Exothermic And Exergonic Chemistry Stack Exchange |

Posting Komentar untuk "are endergonic reactions spontaneous"